Cerdelga is supported by the largest clinical trial program in Gaucher disease type 1 (GD1)
*There were a total of 393 patients with GD1 enrolled across 4 different clinical trials.

Find the information that interests you
New to treatment?
Explore data about patients starting their Cerdelga journey who were previously untreated.
Considering a switch in treatment?
See data on how Cerdelga impacted patients switching from an ERT.
Want to know more about bone death in GD1?
Discover some important risk factors for bone death (osteonecrosis) in patients with GD1 who are on treatment (from an observational study).
PREVIOUSLY UNTREATED PATIENTS
ENGAGE study
ENGAGE was a clinical trial for patients with Gaucher disease type 1 (GD1) who were previously untreated.
What the ENGAGE study can tell us about Cerdelga

Cerdelga significantly improved spleen size, liver size, hemoglobin levels, and platelet levels when compared with placebo at 9 months

ENGAGE also evaluated certain bone health measures

How the ENGAGE study was designed:
40 PATIENTS
Everyone (40 patients aged 16 years and older) in the study had GD1
PREVIOUSLY UNTREATED
All patients had not received treatment within the past 6 to 9 months or had never been on treatment
The main goal of the study

Discover if Cerdelga reduced spleen size in patients at 9 months
Other objectives

Learn if Cerdelga changed:
Liver size
Hemoglobin levels
Platelet levels
Additional information from the study

See how Cerdelga impacted certain
bone health measures:
Bone marrow burden
Bone mineral density
Bone pain

Patients were randomly selected to receive Cerdelga or placebo (a sugar pill).
Half of the patients received Cerdelga (n=20) and the other half received placebo (n=20).
The study was double-blind. This meant that neither doctors nor patients knew which treatment patients received (Cerdelga or placebo).

The first part of the study lasted
9 months (primary analysis).
Patients were observed for up to 4.5 years (open-label extension).

After the first 9 months, most patients joined the long-term phase of the study (open-label extension).
In the open-label extension phase, all patients knew they were taking Cerdelga (no longer blinded).
Every patient who received placebo during the first 9 months received Cerdelga during the extension phase.

Cerdelga significantly improved organ and blood measurements in previously untreated patients.
-
Patients who took Cerdelga showed significant improvements in all of the following measures compared with those on placebo

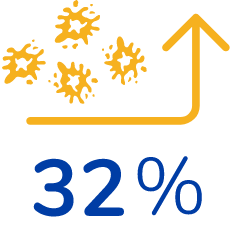
28% reduction in spleen size*

5.2% reduction in liver size*
0.7 g/dL increase in hemoglobin levels*

32% increase in platelet levels*
- Patients who took the placebo had an increase in spleen size of 2.3% and an increase in liver size of 1.4%
- Patients who took the placebo had a decrease in hemoglobin of 0.5 g/dL and a decrease in platelet levels of 9.1%
*From their baseline (pre-treatment) values, on average. Baseline levels were similar between both groups. 20 patients were given Cerdelga and 20 patients were given a sugar pill (placebo).
Patients who took Cerdelga showed significant improvements in all of the following measures compared with those on placebo

28% reduction in spleen size*

5.2% reduction in liver size*
0.7 g/dL increase in hemoglobin levels*

32% increase in platelet levels*
- Patients who took the placebo had an increase in spleen size of 2.3% and an increase in liver size of 1.4%
- Patients who took the placebo had a decrease in hemoglobin of 0.5 g/dL and a decrease in platelet levels of 9.1%
*From their baseline (pre-treatment) values, on average. Baseline levels were similar between both groups. 20 patients were given Cerdelga and 20 patients were given a sugar pill (placebo).
-
At 2 years, patients taking Cerdelga showed the following changes in spleen size, liver size, hemoglobin levels, and platelet levels

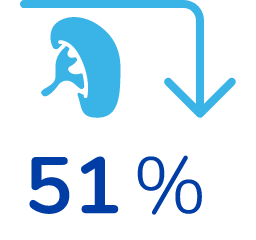
51% reduction in spleen size*

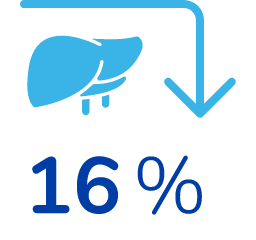
16% reduction in liver size*
1.3 g/dL increase in hemoglobin levels*

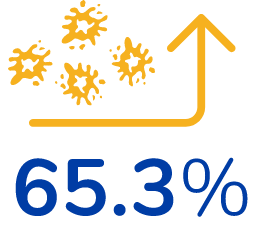
65.3% increase in platelet levels*
- Patients were observed for up to 4.5 years
*From their baseline (pre-treatment) values, on average. Baseline levels were similar between both groups. 38 patients were evaluated.
At 2 years, patients taking Cerdelga showed the following changes in spleen size, liver size, hemoglobin levels, and platelet levels

51% reduction in spleen size*

16% reduction in liver size*
1.3 g/dL increase in hemoglobin levels*

65.3% increase in platelet levels*
- Patients were observed for up to 4.5 years
*From their baseline (pre-treatment) values, on average. Baseline levels were similar between both groups. 38 patients were evaluated.

Additional data from the ENGAGE study
ENGAGE provided additional information about how Cerdelga affects previously untreated patients. This includes some information about bone health. Please note that patients who experienced bone disease symptoms within 12 months before entering the study could not participate in this study.
However, since the study was not designed to assess how Cerdelga impacts bone health, the information is not as reliable as the primary data. Therefore, no conclusions may be drawn regarding the effectiveness of Cerdelga on these measurements or on fracture risk.
CERDELGA AND BONE HEALTH
See how Cerdelga impacted certain bone health assessments in patients new to treatment
-
Cerdelga patients had a reduction in bone marrow burden (BMB) score over 4.5 years
What does this measurement mean?
BMB score is an MRI-based assessment that has been validated as a measurement of a patient’s bone marrow health and treatment response for GD1.How to interpret BMB scores
Mild: 0-4
Moderate: 5-8
Marked to severe: 9-16Due to the lower number of study participants, data after 2 years are not as robust or reliable. Some patients in the ENGAGE study received Cerdelga for up to 4.5 years. For other patients, the trial concluded before they reached 4.5 years. Complete data are not available for the patients who withdrew from the trial.
SD=standard deviation.
Cerdelga patients had a reduction in bone marrow burden (BMB) score over 4.5 years
What does this measurement mean?
BMB score is an MRI-based assessment that has been validated as a measurement of a patient’s bone marrow health and treatment response for GD1.How to interpret BMB scores
Mild: 0-4
Moderate: 5-8
Marked to severe: 9-16Due to the lower number of study participants, data after 2 years are not as robust or reliable. Some patients in the ENGAGE study received Cerdelga for up to 4.5 years. For other patients, the trial concluded before they reached 4.5 years. Complete data are not available for the patients who withdrew from the trial.
SD=standard deviation.
-
Cerdelga patients had an increase in lower spine bone mineral density (BMD) Z-score over 4.5 years
What does this measurement mean?
Lumbar spine BMD Z-score is the difference between a patient’s BMD and the average BMD for people of the same sex and age in the lower back bone.How to interpret Z-scores:
Normal: > -2
Below normal: ≤ -2Due to the lower number of study participants, data after 2 years are not as robust or reliable. Some patients in the ENGAGE study received Cerdelga for up to 4.5 years. For other patients, the trial concluded before they reached 4.5 years. Complete data are not available for the patients who withdrew from the trial.
Lumbar spine=lower back bone; SEM=standard error of the mean.
Cerdelga patients had an increase in lower spine bone mineral density (BMD) Z-score over 4.5 years
What does this measurement mean?
Lumbar spine BMD Z-score is the difference between a patient’s BMD and the average BMD for people of the same sex and age in the lower back bone.How to interpret Z-scores:
Normal: > -2
Below normal: ≤ -2Due to the lower number of study participants, data after 2 years are not as robust or reliable. Some patients in the ENGAGE study received Cerdelga for up to 4.5 years. For other patients, the trial concluded before they reached 4.5 years. Complete data are not available for the patients who withdrew from the trial.
Lumbar spine=lower back bone; SEM=standard error of the mean.
-
Cerdelga patients had an increase in bone mineral density (BMD) Z-score in the thigh bone over 4.5 years

What does this measurement mean?
Femur BMD Z-score is the difference between a patient’s BMD and the average BMD for people of the same sex and age in the thigh bone.How to interpret Z-scores:
Normal: > -2
Below normal: ≤ -2Due to the lower number of study participants, data after 2 years are not as robust or reliable. Some patients in the ENGAGE study received Cerdelga for up to 4.5 years. For other patients, the trial concluded before they reached 4.5 years. Complete data are not available for the patients who withdrew from the trial.
Femur=thigh bone; SEM=standard error of the mean.
Cerdelga patients had an increase in bone mineral density (BMD) Z-score in the thigh bone over 4.5 years

What does this measurement mean?
Femur BMD Z-score is the difference between a patient’s BMD and the average BMD for people of the same sex and age in the thigh bone.How to interpret Z-scores:
Normal: > -2
Below normal: ≤ -2Due to the lower number of study participants, data after 2 years are not as robust or reliable. Some patients in the ENGAGE study received Cerdelga for up to 4.5 years. For other patients, the trial concluded before they reached 4.5 years. Complete data are not available for the patients who withdrew from the trial.
Femur=thigh bone; SEM=standard error of the mean.
-
Almost 80% of patients reported no bone pain at the beginning of the study and almost 80% reported no bone pain at follow-up

What does this measurement mean?
Bone pain was measured using a self-reported GD1-specific bone pain assessment.Patients selected their pain from a scale that ranged from “No pain” to “Extreme pain.”
- Bone pain information from ENGAGE was pulled from patients who were still in the trial at 2.5 years and may not represent the experience of all patients enrolled in the study
- No patient had a bone crisis on Cerdelga and 1 patient taking placebo had a bone crisis
Almost 80% of patients reported no bone pain at the beginning of the study and almost 80% reported no bone pain at follow-up

What does this measurement mean?
Bone pain was measured using a self-reported GD1-specific bone pain assessment.Patients selected their pain from a scale that ranged from “No pain” to “Extreme pain.”
- Bone pain information from ENGAGE was pulled from patients who were still in the trial at 2.5 years and may not represent the experience of all patients enrolled in the study
- No patient had a bone crisis on Cerdelga and 1 patient taking placebo had a bone crisis
SAFETY
Most common side effects (occurring in ≥10% patients) of Cerdelga
|
SIDE EFFECT |
CERDELGA (n=20) |
PLACEBO (n=20) |
|||||||||
|
Number of patients (%) |
Number of patients (%) |
||||||||||
|
Joint Pain |
9 (45%) |
2 (10%) |
|||||||||
|
Headache |
8 (40%) |
6 (30%) |
|||||||||
|
Migraine |
2 (10%) |
0 (0%) |
|||||||||
|
Flatulence |
2 (10%) |
1 (5%) |
|||||||||
|
Nausea |
2 (10%) |
1 (5%) |
|||||||||
|
Mouth Pain |
2 (10%) |
1 (5%) |
|
SIDE EFFECT |
Number of patients (%) |
||||||||||
|
Joint Pain |
9 (45%) |
2 (10%) |
|||||||||
|
Headache |
8 (40%) |
6 (30%) |
|||||||||
|
Migraine |
2 (10%) |
0 (0%) |
|||||||||
|
Flatulence |
2 (10%) |
1 (5%) |
|||||||||
|
Nausea |
2 (10%) |
1 (5%) |
|||||||||
|
Mouth Pain |
2 (10%) |
1 (5%) |
|||||||||
- 0 patients discontinued the study due to side effects in the primary analysis period
- No new side effects were onserved in the long-term extension period
n=sample size.

These are not all the possible side effects of Cerdelga

Call your doctor for medical advice about side effects
PATIENTS SWITCHING FROM ERT
ENCORE study
The ENCORE study was a clinical trial for patients with Gaucher disease type 1 (GD1) who were stable on an enzyme replacement therapy (ERT). The study evaluated whether Cerdelga was as effective as an ERT.
What the ENCORE study can tell us about Cerdelga

Organ and blood measurements remained stable in patients taking Cerdelga

Cerdelga is just as
effective as an ERT

ENCORE also evaluated certain bone health measures

How the ENCORE study was designed:
159 PATIENTS
Everyone (159 patients aged 18 or older) in the study had GD1
STABLE ON ERT
All patients were stable on ERT
The main goal of the study

See if patients switching to Cerdelga did as well as patients remaining on ERT
For the study to meet its main goal

Patients needed to remain stable on all the following measures:
Spleen size
Liver size
Platelet levels
Hemoglobin levels
Additional information from the study

See how Cerdelga impacted certain bone health measures:
Bone marrow burden
Bone mineral density
Bone pain

Patients were randomly selected to receive Cerdelga or an ERT (imiglucerase).
For every 2 patients who received Cerdelga, 1 patient received imiglucerase (106 patients were taking Cerdelga and 53 were taking imiglucerase).
The study was open label. This meant that both doctors and patients knew which treatment the patients received.

The first part of the study lasted
12 months (primary analysis).
Patients were observed for up to
4 years (open-label extension).

After the first 12 months, most patients joined the long-term phase of the study (open-label extension).
141 of the 146 patients from the primary phase of the ENCORE trial were evaluated long term. This included 42 previously treated with an ERT and 99 who continued treatment with Cerdelga.
Most patients switching from ERT to Cerdelga remained stable
-
ENCORE successfully showed that Cerdelga is just as effective as an ERT

Spleen size

Liver size

Hemoglobin levels

Platelet levels
- There were no clinically meaningful differences between patients receiving Cerdelga versus ERT for any of the 4 measures
- Patients on Cerdelga remained stable in all 4 measurements after switching from ERT at 12 months
- 85% of patients on Cerdelga remained stable after switching from ERT compared to 94% of patients who remained on ERT
ENCORE successfully showed that Cerdelga is just as effective as an ERT

Spleen size

Liver size

Hemoglobin levels

Platelet levels
- There were no clinically meaningful differences between patients receiving Cerdelga versus ERT for any of the 4 measures
- Patients on Cerdelga remained stable in all 4 measurements after switching from ERT at 12 months
- 85% of patients on Cerdelga remained stable after switching from ERT compared to 94% of patients who remained on ERT
-
At 2 years, in the extension phase of the study, most patients taking long-term, open-label Cerdelga remained stable

Spleen size

Liver size

Hemoglobin
levels
Platelet
levels- The percentages of patients who were stable in spleen and liver volume, hemoglobin level, and platelet count were 120/141 (85%) after 1 year and 111/129 (86%) after 2 years of Cerdelga treatment
- Patients were observed for up to 4 years
At 2 years, in the extension phase of the study, most patients taking long-term, open-label Cerdelga remained stable

Spleen size

Liver size

Hemoglobin
levels
Platelet
levels- The percentages of patients who were stable in spleen and liver volume, hemoglobin level, and platelet count were 120/141 (85%) after 1 year and 111/129 (86%) after 2 years of Cerdelga treatment
- Patients were observed for up to 4 years

Additional data from the ENCORE study
ENCORE provided additional information about how patients switching from an ERT to Cerdelga were affected. This includes some information about bone health. Please note that patients who experienced bone disease symptoms within 12 months before entering the study could not participate in this study.
However, since the study was not designed to assess how Cerdelga impacts bone health, the information is not as reliable as the primary data. Therefore, no conclusions may be drawn regarding the effectiveness of Cerdelga on these measurements or on fracture risk.
CERDELGA AND BONE HEALTH
See how Cerdelga impacted certain bone health assessments in patients who switched from an ERT
-
Cerdelga patients who switched from ERT had a stable bone marrow burden (BMB) score in the moderate range over 4 years

What does this measurement mean?
BMB score is an MRI-based assessment that has been validated as a measurement of a patient’s bone marrow health and treatment response for GD1.How to interpret BMB scores
Mild: 0-4
Moderate: 5-8
Marked to severe: 9-16Due to the lower number of study participants, data after 2 years are not as robust or reliable. Some patients in the ENCORE study received Cerdelga for up to 4 years. For other patients, the trial concluded before they reached 4 years. Complete data are not available for the patients who withdrew from the trial.
SEM=standard error of the mean.
Cerdelga patients who switched from ERT had a stable bone marrow burden (BMB) score in the moderate range over 4 years

What does this measurement mean?
BMB score is an MRI-based assessment that has been validated as a measurement of a patient’s bone marrow health and treatment response for GD1.How to interpret BMB scores
Mild: 0-4
Moderate: 5-8
Marked to severe: 9-16Due to the lower number of study participants, data after 2 years are not as robust or reliable. Some patients in the ENCORE study received Cerdelga for up to 4 years. For other patients, the trial concluded before they reached 4 years. Complete data are not available for the patients who withdrew from the trial.
SEM=standard error of the mean.
-
Cerdelga patients who switched from ERT had stable bone mineral density (BMD) scores in the lower back bone over 4 years

What does this measurement mean?
Lumbar spine BMD Z-score is the difference between a patient’s BMD and the average BMD for people of the same sex and age in the lower back bone.How to interpret Z-scores:
Normal: > -2
Below normal: ≤ -2Due to the lower number of study participants, data after 2 years are not as robust or reliable. Some patients in the ENCORE study received Cerdelga for up to 4 years. For other patients, the trial concluded before they reached 4 years. Complete data are not available for the patients who withdrew from the trial.
Lumbar spine=lower back bone; SEM=standard error of the mean.
Cerdelga patients who switched from ERT had stable bone mineral density (BMD) scores in the lower back bone over 4 years

What does this measurement mean?
Lumbar spine BMD Z-score is the difference between a patient’s BMD and the average BMD for people of the same sex and age in the lower back bone.How to interpret Z-scores:
Normal: > -2
Below normal: ≤ -2Due to the lower number of study participants, data after 2 years are not as robust or reliable. Some patients in the ENCORE study received Cerdelga for up to 4 years. For other patients, the trial concluded before they reached 4 years. Complete data are not available for the patients who withdrew from the trial.
Lumbar spine=lower back bone; SEM=standard error of the mean.
-
Cerdelga patients who switched from ERT had stable bone mineral density (BMD) scores in the thigh bone over 4 years

What does this measurement mean?
Femur BMD Z-score is the difference between a patient’s BMD and the average BMD for people of the same sex and age in the thigh bone.How to interpret Z-scores:
Normal: > -2
Below normal: ≤ -2Due to the lower number of study participants, data after 2 years are not as robust or reliable. Some patients in the ENCORE study received Cerdelga for up to 4 years. For other patients, the trial concluded before they reached 4 years. Complete data are not available for the patients who withdrew from the trial.
Femur=thigh bone; SEM=standard error of the mean.
Cerdelga patients who switched from ERT had stable bone mineral density (BMD) scores in the thigh bone over 4 years

What does this measurement mean?
Femur BMD Z-score is the difference between a patient’s BMD and the average BMD for people of the same sex and age in the thigh bone.How to interpret Z-scores:
Normal: > -2
Below normal: ≤ -2Due to the lower number of study participants, data after 2 years are not as robust or reliable. Some patients in the ENCORE study received Cerdelga for up to 4 years. For other patients, the trial concluded before they reached 4 years. Complete data are not available for the patients who withdrew from the trial.
Femur=thigh bone; SEM=standard error of the mean.
-
Among patients who switched from ERT to Cerdelga, almost 70% reported no bone pain at baseline and nearly 90% reported no bone pain at 4 years

What does this measurement mean?
Bone pain was measured using a self-reported GD1-specific bone pain assessment.Patients selected their pain from a scale that ranged from “No pain” to “Extreme pain.”
- Bone pain information from ENCORE was pulled from patients who were still in the trial at 4 years and may not represent the experience of all patients enrolled in the study
- In the ENCORE trial, 3 patients (2%) had a bone crisis while taking Cerdelga and 1 patient on ERT had a bone crisis
Among patients who switched from ERT to Cerdelga, almost 70% reported no bone pain at baseline and nearly 90% reported no bone pain at 4 years

What does this measurement mean?
Bone pain was measured using a self-reported GD1-specific bone pain assessment.Patients selected their pain from a scale that ranged from “No pain” to “Extreme pain.”
- Bone pain information from ENCORE was pulled from patients who were still in the trial at 4 years and may not represent the experience of all patients enrolled in the study
- In the ENCORE trial, 3 patients (2%) had a bone crisis while taking Cerdelga and 1 patient on ERT had a bone crisis
SAFETY
Most common side effects (occurring in ≥5% patients) of Cerdelga
|
SIDE EFFECT |
CERDELGA (n=20) |
ERT (n=53) |
|||||||||
|
Number of patients (%) |
Number of patients (%) |
||||||||||
|
Fatigue |
15 (14%) |
1 (2%) |
|||||||||
|
Headache |
14 (13%) |
1 (2%) |
|||||||||
|
Nausea |
13 (12%) |
0 (0%) |
|||||||||
|
Diarrhea |
13 (12%) |
2 (4%) |
|||||||||
|
Back Pain |
13 (12%) |
3 (6%) |
|||||||||
|
Pain in Extremity |
12 (11%) |
1 (2%) |
|||||||||
|
Upper Abdominal Pain |
11 (10%) |
0 (0%) |
|||||||||
|
Dizziness |
9 (8%) |
0 (0%) |
|||||||||
|
Weakness |
9 (8%) |
0 (0%) |
|||||||||
|
Cough |
7 (7%) |
2 (4%) |
|||||||||
|
Indigestion |
7 (7%) |
1 (2%) |
|||||||||
|
Heartburn |
7 (7%) |
0 (0%) |
|||||||||
|
Constipation |
5 (5%) |
0 (0%) |
|||||||||
|
Racing Heart |
5 (5%) |
0 (0%) |
|||||||||
|
Rash |
5 (5%) |
0 (0%) |
|
SIDE EFFECT |
Number of patients (%) |
||||||||||
|
Fatigue |
15 (14%) |
1 (2%) |
|||||||||
|
Headache |
14 (13%) |
1 (2%) |
|||||||||
|
Nausea |
13 (12%) |
0 (0%) |
|||||||||
|
Diarrhea |
13 (12%) |
2 (4%) |
|||||||||
|
Back Pain |
13 (12%) |
3 (6%) |
|||||||||
|
Pain in Extremity |
12 (11%) |
1 (2%) |
|||||||||
|
Upper Abdominal Pain |
11 (10%) |
0 (0%) |
|||||||||
|
Dizziness |
9 (8%) |
0 (0%) |
|||||||||
|
Weakness |
9 (8%) |
0 (0%) |
|||||||||
|
Cough |
7 (7%) |
2 (4%) |
|||||||||
|
Indigestion |
7 (7%) |
1 (2%) |
|||||||||
|
Heartburn |
7 (7%) |
0 (0%) |
|||||||||
|
Constipation |
5 (5%) |
0 (0%) |
|||||||||
|
Racing Heart |
5 (5%) |
0 (0%) |
|||||||||
|
Rash |
5 (5%) |
0 (0%) |
|||||||||
- Two patients given Cerdelga discontinued in the ENCORE study due to side effects
- No new side effects were observed in the long-term extension study
n=sample size.

These are not all the possible side effects of Cerdelga

Call your doctor for medical advice about side effects
RISKS OF BONE DEATH IN PATIENTS WITH GD1 RECEIVING TREATMENT
20-year observational study
Bone tissue death, or osteonecrosis, is one of the most severe and debilitating symptoms of GD1. Any patient with GD1 may be at risk for bone death, even while on treatment.
What the observational study can tell us about bone death and treatment for GD1
This study evaluated what puts patients with GD1 at risk for bone death. The study focused on patients with GD1 receiving treatment.
How the observational study was designed:
155 PATIENTS
155 patients with GD1 at a single center
ON TREATMENT
All patients were on treatment for GD1 (ERT or Cerdelga)
The main goal of the study

Evaluate the risk factors for bone death (osteonecrosis) among patients with GD1 receiving treatment
Patients could only be included in the study if there was:

A confirmed diagnosis of GD1
An accurate treatment history and medical history
Limitations of data from this observational, retrospective study:
This study was observational and retrospective. It was not a clinical trial. The study was not designed to compare one treatment to another. Patients were not assigned different treatment types randomly, as they would be in many clinical trials. Instead, treatment decisions were made by their physician based on many different factors, including the patient’s GD1 status and what treatment options were available. It is possible that patients who received one type of treatment had more severe GD1 symptoms than patients who received a different type of treatment.

No patients taking Cerdelga experienced irreversible bone tissue death in this observational 20-year study.

During this 20-year period, some patients may
have switched treatments.
There are many factors that impact bone
death. It is unclear exactly why 0 patients on
Cerdelga experienced bone death during this
study.
- There were 16 episodes of bone death in 14 patients (2 patients each experienced 2 episodes)
- For Cerdelga and velaglucerase alfa, data are only available starting from 2014 and 2010, respectively, when they were approved by the FDA
4 risk factors for bone death in patients with GD1 receiving treatment
This observational study found the following factors put patients with GD1 at increased risk of bone death.
A treatment history with a type of ERT

Increased levels of GD1-associated compounds in the blood*

Certain genetic markers

History of bone death prior to starting treatment
*This study found that all three therapy options reduced the amount of lyso-GL-1 and the amount of chitotriosidase in patients with GD1. The study found patients who had persistently high levels of lyso-GL-1, even after starting GD1 therapy, had an increased risk for bone death (osteonecrosis). The study did not find the same association between high levels of chitotriosidase after starting GD1 therapy and the risk of bone death in this group of patients.

Talk to your healthcare provider about your potential risk factors.
Ready to start Cerdelga?
Most adult patients with GD1 are eligible for Cerdelga.
Need support along your journey?
Sanofi is committed to supporting patients with GD1.
Indication
CERDELGA is a prescription medicine used for the long-term treatment of Gaucher disease type 1 (GD1) in adults who are CYP2D6 extensive metabolizers (EMs), intermediate metabolizers (IMs), or poor metabolizers (PMs) as detected by an FDA-cleared test. Your doctor will perform a test to make sure that CERDELGA is right for you.
Limitations of Use:
- CYP2D6 ultra-rapid metabolizers may not achieve adequate concentrations of CERDELGA to achieve a therapeutic effect.
- A specific dose cannot be recommended for CYP2D6 indeterminate metabolizers.
Important Safety Information
Certain patients should not use CERDELGA based on their CYP2D6 metabolizer status due to an increased risk of side effects, including heart problems. Do not use CERDELGA if you are:
- An Extensive Metabolizer (EM) taking a medicine that is a strong or moderate CYP2D6 inhibitor along with another medicine that is a strong or moderate CYP3A inhibitor, an EM with moderate or severe liver problems, or an EM with mild liver problems and taking a medicine that is a strong or moderate CYP2D6 inhibitor.
- An Intermediate Metabolizer (IM) taking a medicine that is a strong or moderate CYP2D6 inhibitor along with another medicine that is a strong or moderate CYP3A inhibitor, an IM taking a medicine that is a strong CYP3A inhibitor, or an IM with any degree of liver problems.
- A Poor Metabolizer (PM) taking a medicine that is a strong CYP3A inhibitor, or a PM with any degree of liver problems.
Your doctor will perform a test to help determine if CERDELGA is right for you.
CERDELGA can affect the way other medicines work and other medicines can affect how CERDELGA works. Using CERDELGA with other medicines or herbal supplements may cause an increased risk of side effects, including changes in electrical activity of your heart (ECG changes) and irregular heart beat (arrhythmias). Especially tell your doctor if you take St. John's Wort, or medicines for fungal infections, tuberculosis, seizures, heart conditions, high blood pressure, or depression or other mental health problems. Your doctor may need to prescribe a different medicine, change your dose of other medicines, or change your dose of CERDELGA. Tell your doctor about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements before you start taking them.
Before taking CERDELGA, tell your doctor about all of your medical conditions, including heart problems (including a condition called long QT syndrome), a history of heart attack, kidney or liver problems. If you are pregnant or plan to become pregnant or breastfeed, talk to your doctor. It is not known if CERDELGA will harm your unborn baby. Talk to your doctor if you are breastfeeding or planning to breastfeed. It is not known if CERDELGA passes into your breast milk. You and your doctor will decide if you should take CERDELGA or breastfeed. You should not do both.
CERDELGA, used with certain other medicines, may cause changes in the electrical activity of your heart (ECG changes) and irregular heart beat (arrhythmias). Tell your doctor if you have new symptoms such as palpitations, fainting, or dizziness.
The most common side effects (≥10%) of CERDELGA include: tiredness, headache, nausea, diarrhea, back pain, pain in extremities, and upper abdominal pain. Call your doctor for medical advice about adverse effects.
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of CERDELGA. Call your doctor for medical advice about side effects.
It is not known if CERDELGA is safe and effective in children.
Please see the full Prescribing Information, including the Patient Medication Guide, for CERDELGA.
Indication
CERDELGA is a prescription medicine used for the long-term treatment of Gaucher disease type 1 (GD1) in adults who are CYP2D6 extensive metabolizers (EMs), intermediate metabolizers (IMs), or poor metabolizers (PMs) as detected by an FDA-cleared test. Your doctor will perform a test to make sure that CERDELGA is right for you.
Limitations of Use:
- CYP2D6 ultra-rapid metabolizers may not achieve adequate concentrations of CERDELGA to achieve a therapeutic effect.
- A specific dose cannot be recommended for CYP2D6 indeterminate metabolizers.
Important Safety Information
Certain patients should not use CERDELGA based on their CYP2D6 metabolizer status due to an increased risk of side effects, including heart problems. Do not use CERDELGA if you are:
- An Extensive Metabolizer (EM) taking a medicine that is a strong or moderate CYP2D6 inhibitor along with another medicine that is a strong or moderate CYP3A inhibitor, an EM with moderate or severe liver problems, or an EM with mild liver problems and taking a medicine that is a strong or moderate CYP2D6 inhibitor.
- An Intermediate Metabolizer (IM) taking a medicine that is a strong or moderate CYP2D6 inhibitor along with another medicine that is a strong or moderate CYP3A inhibitor, an IM taking a medicine that is a strong CYP3A inhibitor, or an IM with any degree of liver problems.
- A Poor Metabolizer (PM) taking a medicine that is a strong CYP3A inhibitor, or a PM with any degree of liver problems.
Your doctor will perform a test to help determine if CERDELGA is right for you.
CERDELGA can affect the way other medicines work and other medicines can affect how CERDELGA works. Using CERDELGA with other medicines or herbal supplements may cause an increased risk of side effects, including changes in electrical activity of your heart (ECG changes) and irregular heart beat (arrhythmias). Especially tell your doctor if you take St. John's Wort, or medicines for fungal infections, tuberculosis, seizures, heart conditions, high blood pressure, or depression or other mental health problems. Your doctor may need to prescribe a different medicine, change your dose of other medicines, or change your dose of CERDELGA. Tell your doctor about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements before you start taking them.
Before taking CERDELGA, tell your doctor about all of your medical conditions, including heart problems (including a condition called long QT syndrome), a history of heart attack, kidney or liver problems. If you are pregnant or plan to become pregnant or breastfeed, talk to your doctor. It is not known if CERDELGA will harm your unborn baby. Talk to your doctor if you are breastfeeding or planning to breastfeed. It is not known if CERDELGA passes into your breast milk. You and your doctor will decide if you should take CERDELGA or breastfeed. You should not do both.
CERDELGA, used with certain other medicines, may cause changes in the electrical activity of your heart (ECG changes) and irregular heart beat (arrhythmias). Tell your doctor if you have new symptoms such as palpitations, fainting, or dizziness.
The most common side effects (≥10%) of CERDELGA include: tiredness, headache, nausea, diarrhea, back pain, pain in extremities, and upper abdominal pain. Call your doctor for medical advice about adverse effects.
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of CERDELGA. Call your doctor for medical advice about side effects.
It is not known if CERDELGA is safe and effective in children.
Please see the full Prescribing Information, including the Patient Medication Guide, for CERDELGA.